Sosnovtseva Group - Biophysics and Biosimulation
We combine innovative experimental techniques, new methods of data analysis and mechanism-based modeling to unravel complex relationships between structure, dynamics, and function of cellular and vascular networks in both health and disease.
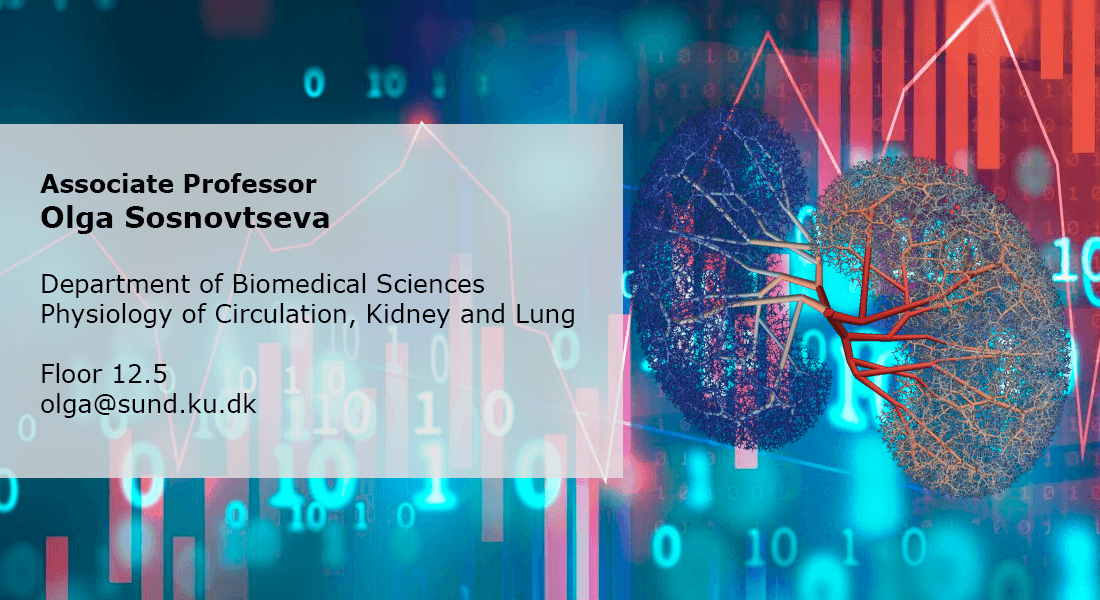
We aim to understand the complex interplay between renal autoregulatory mechanisms and causes of their pathological changes related to hypertension and diabetes. We use integrated approach: we combine novel experimental techniques, advanced machine learning computational algorithms, and modeling on macroscopic and microscopic levels. With this, we map topological and dynamical features of renal autoregulation within the framework of translational studies.
Mitochondrial dysfunction is involved in a range of pathological conditions, including cardiovascular diseases, neurodegeneration, diabetes, and cancer. There is no technique for direct, label-free, non-invasive monitoring of the state of mitochondria in living organs. We develop a non-invasive approach based on Raman spectroscopy to assess redox state of the electron transport chain of mitochondria in living cells, tissues, and organs.
In vivo methods
We measure blood pressure, renal blood flow, glomerular filtration, urine excretion rate, composition of blood and urine in rodents under different experimental conditions:
- Changes in blood pressure
- Infusion of pharmacological substances (acute)
- Treatment over longer periods (chronic)Laser speckle flowmetry
We assess microcirculatory hemodynamics of the kidney cortex of anesthetized rats to evaluate the efficiency of autoregulatory mechanisms.
Modeling and data analysis
We use mechanism-based and data driven modeling.
We use image analysis in time-frequency domain.
Raman spectroscopy
We use Raman spectroscopy that is a non-invasive technique providing highly specific information on the biochemical composition and molecular structure of samples.
Virtual kidney

The renal vasculature, acting as a resource distribution network, plays an important role in the physiology and pathophysiology of the kidney. However, no imaging technique allows a simultaneous assessment of the structural, dynamical, and functional features of the renal vasculature, because of limited spatial and temporal resolution. We build a full-scale model of the kidney to develop realistic computer simulations of renal function and to develop new image-based diagnostic methods based on artificial intelligence.
Renal microcirculatory hemodynamics
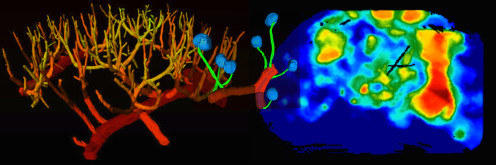
Diabetes is a large and growing health problem and a primary cause of chronic kidney disease. The mechanisms behind chronic kidney disease are not clear. New treatments with SGLT2 inhibitors control blood glucose in patients with diabetes by reducing the kidney's uptake of glucose by blocking tits co-transport with sodium. These treatments significantly improve kidney function. However, their reno-protective effect is not understood yet. We examine the acute and chronic renal effects of SGLT2 inhibitors in different animal models with focus on renal autoregulation. By using the latest imaging techniques, we will investigate how the TGF-induced vascular responses change in diabetes and whether these changes are prevented by SGLT2 inhibitors.
Optical fingerprint of mitochondria
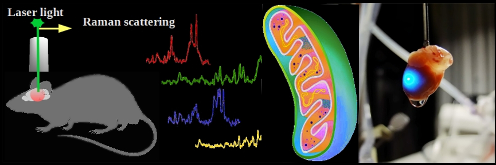
At present, widely-used methods for the quantification of electron transport chain activity in mitochondria are biochemical techniques that measure respiration rate of mitochondria and the amount of certain complexes. We propose to illuminate the molecules of the mitochondrial electron-transport chain with laser light to detect, record, and interpret their optical fingerprints – a unique set of bond-vibrational energies providing information about redox state and conformation of electron-transport chain components. By appropriate choice the excitation wavelength, one can achieve Raman scattering predominantly from different cytochromes. This is the only technique that can assess mitochondrial redox state in living organs in vivo.
2023–2026 PI, Novo Nordisk Foundation, Renoprotective effects of sodium-glucose co-transporter inhibitors: Role of the tubuloglomerular feedback mechanism
2020–2024 PI, UCPH Data+ pool UCPH Strategy 2023, Virtual kidney
2020 PI, UCPH Strategy 2023 grant research-integrating teaching activities, Physiological modeling computer cluster PIONEER
2019–2022 PI, Novo Nordisk Foundation, Renal autoregulation in health and disease: New insights into topology and dynamics
Group Leader
Olga Sosnovtseva
Associate Professor
Phone +45 35 32 74 69
olga@sund.ku.dk
ORCID: 0000-0001-7334-9869
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Oleg Zhukov | Postdoc |

