Jacobsen Group - Vascular Modeling
Research in the Vascular Modeling Laboratory is focused on integrative models of the microcirculation. We apply multiple-scale computational models to investigate basic mechanisms operating in the microcirculation in both health and disease.
Ultimately, the aim of the group's research is to contribute to the body of knowledge necessary to develop new treatment strategies for conditions such as diabetes and hypertension which affects the microcirculation.
We are interested in the dynamics of small molecules and ions including Ca2+ in restricted areas just below the cell membrane. The concentration of Ca2+ in such microdomains may vastly exceed that found in the rest of the cell in turn this may initiate specific reactions and modulate communication in the vascular wall. Microdomain mechanisms may also operate in the tubuli of the kidney in relation to blood pressure regulation.
On an intermediary length scale we apply models of vascular wall cells that incorporate cellular electrophysiology and intercellular coupling through gap junctions. Coupling of numerous cells into a vessel-like structure results in emergent properties such as intercellular synchronization and intercellular spread of electrical signals, so-called vascular conducted responses.
At the largest length scale we work with the properties of microvascular networks - how networks remodel and change in hypertension and how the retinal microcirculation behaves under various conditions.
The Vascular Modeling Laboratory focuses on four research areas described below.
Ion concentrations in microdomains
There are many unresolved questions in human biology. One of them is the regulation of the processes that takes place very close to the membrane.
The plasma membrane that surrounds the cell consists of a complex mixture of phospholipids, proteins and other molecules. The plasma membrane is packed with ion channels and ion transporters which regulate the ion gradients and the intracellular concentrations. Proteins that form the cell skeleton and control shape changes are also anchored in the plasma membrane.
Changes in the conformation and activity of these proteins are often regulated by Ca2+, however, in vitro studies often show that Ca2+ concentrations of 10-100 µM are required whereas Ca2+ in the bulk cytosol is between 10 nM - 1 µM. There is a huge gap between these numbers, which often is unexplained. Recent studies have shown that very high concentrations of e.g. Ca2+ can be found in microdomains close to the cell membrane.
My research is focused on the ion concentrations in microdomains close to the cell membrane and how they affect the cell and the entire organism. I focus on two systems namely reabsorption in the proximal tubuli in the kidney and formation of Ca2+ microdomains in endothelial and smooth muscle cells in the vessels.
Get to know more about the research area by contacting Jens Christian Brings.
Vascular and cellular networks
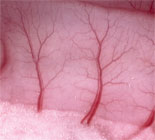 Spontaneous onset of intercellular synchronization in smooth muscle cells of the arteriolar wall underlies the transient phenomenon known a vasomotion. Since synchronization is a hallmark of smooth muscle tissue in many organs, the vascular wall may serve as a model tissue in this respect and thus we have been interested in modeling the synchronization process in detail.
Spontaneous onset of intercellular synchronization in smooth muscle cells of the arteriolar wall underlies the transient phenomenon known a vasomotion. Since synchronization is a hallmark of smooth muscle tissue in many organs, the vascular wall may serve as a model tissue in this respect and thus we have been interested in modeling the synchronization process in detail.
Hypertension, in both mild and severe forms, is a major cause of cardiovascular disease including heart disease and stroke. The microcirculatory bed in hypertension is characterized by specific morphological changes. In essential hypertension these changes include vascular remodeling. The latter may however, represent a somewhat pathological result of a normo-physiological adaptation potential ubiquitously present in our microcirculation. This ability of the microcirculation to adapt is necessary in order for the microcirculation to continuously meet the changing demands of the tissue; hence we are interested in how vascular networks are able to actively change their structure in both normo- and hypertension.
In essential hypertension these changes include vascular remodeling. The latter may however, represent a somewhat pathological result of a normo-physiological adaptation potential ubiquitously present in our microcirculation. This ability of the microcirculation to adapt is necessary in order for the microcirculation to continuously meet the changing demands of the tissue; hence we are interested in how vascular networks are able to actively change their structure in both normo- and hypertension.
In more severe forms of acute hypertension vessels may display a pattern of alternating constricted and dilated areas known as sausage-string formation. The emergence of this pattern has been an enigma for many years - however modeling suggests that it may be due to instability of the vascular wall present during high levels of smooth muscle activation.
Get to know more about the research area by contacting Jens Christian Brings Jacobsen, Associate professor.
Intercellular diffusion
Gap junctions are specialized intercellular channels directly connecting the cytoplasm of adjacent cells. They allow for electrical and metabolic coupling in many kinds of tissues; in the vascular wall they enable conduction of electrical signals conveying information along the vascular tree, and in the heart gap junctions underlie the fast spread of depolarization preceding contraction. In our lab we apply a dye-based assay with the potential to monitor drug-modulating effects on gap junction permeability. The main interest has been in modeling this assay mathematically in order to estimate the permeability in quantitative terms.
Vasomotion is spontaneous oscillation in the tonus of the smooth muscles in the arteriolar walls giving rise to 0.1 Hz diameter fluctuations. Its occurrence is known to correlate with diseases such as hypertension and diabetes. The microcirculation of the retina provides an unique opportunity to observe vasomotion without eksperimental intervention. The current work is in quantifying vasomotion in recordings of the retina by using advanced image analysis, and correlate these findings with the state of a given disease in order to develop new diagnostic and prognostic measures.
Regulation of microvascular tone
Biology appears to be largely characterized by a precise temporal and spatial organization. On the other hand, however, many transient interventions that alter the organisation within a biological system do not induce any significant pathology in the system.
Moreover, upon dissecting similar biological tissue, e.g. heart tissue or a specific vascular bed, the finer biological details seem to be fairly heterogeneous. Thus, biology may also be characterized by an equally high degree of plasticity and robustness. The unfolding of the underlying nature of these phenomena is of great personal interest.
In the vasculature, these features are very clear. The dynamics of signalling systems produce a precise regulation of the vascular beds although the systems are build from an underlying heterogeneous basis. We use a mathematical modelling approach to investigate the dynamics of
- Propagated signalling responses across large distances
- The intercellular synchronization of smooth muscle cells of the arteriolar wall.
Analysis of simulated signalling responses from underlying models of heterogeneous vascular cells may furnish a better understanding of both vascular signalling pathways and the robustness of biological systems as such.
Bjørn Olav Hald is focused on this research area. If you want to know more about Bjørn's research, please contact him.
The vascular modeling lab is involved in several undergraduate and graduate courses given at the Faculty of Health Sciences. Thus, we are involved in the teaching of Medical Engineer-, Health Informatics- and Medical students.
Vice Head of studies
Bachelor's programme in Quantitative Biology and Disease Modeling
Course leader: Human Diseases for Non-Clinicians (10 ECTS)
Departmental supervisor (Teaching)
Courses
- Modeling of Physiological Systems (incl. course leadership)
- Advanced Modeling of Physiological Systems
- Physiology for human biologists (respiratory physiology, gastro-intestinal physiology, hormonal systems, metabolism and reproduction).
- Respiratory Physiology and diseases of the lung (Medicine, Medicine and Technology)
- Hormonal Systems (Molecular Biomedicine)
Supervision
- Supervision of Master-, bachelor-, special-course- and research year students.
- Supervisor and co-supervisor for PhD students
- EU project: PI in the Marie Curie Initial Training Network REVAMMAD. 2013-2017: DKK 2.100.000
- Bonus from UCPH for bringing home EU funding (2013): DKK 500.000
- 2013-2016 Named participant, UCPH's 2016 Funds: Dynamical Systems - Mathematical Modeling and Statistical Methodology for the Social, Health and Natural Sciences.
- EU project: Member of the 2004-2009 EU Network of Excellence Biosim (PI N-H Holstein-Rathlou).
Group Leader
Jens Christian Brings Jacobsen
Associate Professor
Phone +45 3532 7401
jcbrings@sund.ku.dk


