Davies Group - Protein Oxidation
Our group works to understand the mechanisms of protein modification by reactive species (radicals, two-electron oxidants, glycation reactions), the biological consequences of such reactions, and the development of methods to quantify protein damage in disease with a particular emphasis on cardiovascular pathologies.

Proteins are the ‘workers’ of cells and essential for life as a result of their structure and functional roles. They are highly abundant inside and external to cells, but are susceptible to damage by oxidants arising from cellular processes and external factors (e.g. radiation, UV light, pollutants, cigarette smoke, etc).
This oxidative stress, which can accumulate during ageing and be enhanced by inflammation and disease, can result in protein and tissue dysfunction. There is abundant evidence for an accumulation of protein damage in many human diseases, but our knowledge of how this arises, the role that it plays in disease initiation and development, and how to prevent it, is limited.
We want to determine how and when oxidative damage occurs on proteins, in a quantitative and rigorous manner. Such data will allow the rational and informed development of protective strategies against damage and disease.
We are particularly interested in:
- modifications arising from peroxidase enzymes (e.g. myeloperoxidase) and other oxidants,
- methods to detect these species and resulting damage,
- the kinetics of such reactions,
- how these reactions result in damage to cells and their surrounding extracellular matrix,
- the role that these reactions play in cell dysfunction and human disease (particularly atherosclerosis), and
- developing antioxidants and inhibitors to minimize damage.
- Analysis of human carotid and coronary arteries (ex vivo and in vivo) and blood samples
- Animal models of atherosclerosis
- Primary human endothelial and smooth muscle cells and extracellular matrix
- Liquid chromatography-mass spectrometry analysis of proteins (proteomics)
- Biochemical and cellular assays, molecular biology methods (gels, immunoblotting, immunofluorescence, ELISA, BiaCore, enzymatic assays, TEM)
- Kinetic analysis of reactive intermediates and their reactions (stopped flow, fast reaction techniques, UPLC)
- Detection, identification and quantification of oxidative stress markers and biological damage
 Proteins are critical to all aspects of cell and tissue structure and function, but are prone to damage which can result in dysfunction and disease. Damage to proteins can arise from the reactions of radicals (reactive species with an unpaired electron) and other oxidants, which are generated in biological systems by both endogenous processes (e.g. metabolic pathways, inflammation and enzyme reactions) and also exposure to a wide range of external factors (UV and high-energy radiation, exposure to drugs, pollutants, mineral fibres, chemicals etc). Proteins are major targets due to their high abundance and their rapid rates of reaction with these oxidants. Damage to proteins can also arise from proteolytic enzymes that are activated by oxidants.
Proteins are critical to all aspects of cell and tissue structure and function, but are prone to damage which can result in dysfunction and disease. Damage to proteins can arise from the reactions of radicals (reactive species with an unpaired electron) and other oxidants, which are generated in biological systems by both endogenous processes (e.g. metabolic pathways, inflammation and enzyme reactions) and also exposure to a wide range of external factors (UV and high-energy radiation, exposure to drugs, pollutants, mineral fibres, chemicals etc). Proteins are major targets due to their high abundance and their rapid rates of reaction with these oxidants. Damage to proteins can also arise from proteolytic enzymes that are activated by oxidants.
Oxidant-mediated damage has been linked to ageing, human disease, as well as changes in food quality and shelf life, agricultural yields and the quality and efficacy of medicines, vaccines and antibodies.
Current projects
-
Mapping protein changes in human carotid artery atherosclerotic plaques and blood samples by proteomics.
In this study we are examining whether there are significant changes in the complement of proteins present in human atherosclerotic plaques and whether this correlates (and predicts) the stability of these plaques. Rupture of such plaques is a major cause of strokes.
- Novel blood biomarkers of cardiovascular disease
 We are examining whether there are differences in the types and levels of materials released from atherosclerotic plaques into the circulation. Identification of these materials may provide novel biomarkers of cardiovascular disease and predict disease progression.
We are examining whether there are differences in the types and levels of materials released from atherosclerotic plaques into the circulation. Identification of these materials may provide novel biomarkers of cardiovascular disease and predict disease progression.
- Oxidation- and glycation-induced modifications in human carotid artery atherosclerotic plaques.
Increased oxidation and elevated glucose levels are associated with the atherosclerosis. In this project we are identifying and quantifying protein modifications present in human tissue samples. Knowledge of the processes that give rise to damage, may guide new therapeutic strategies.
- Proteomic analysis of proteins on percutaneous coronary intervention (PCI) angioplasty balloons to allow differentiation of subjects with stable versus unstable cardiovascular disease.
We have shown that PCI balloons used to open up blocked arteries retain proteins on their surface which can be identified and quantified by proteomics. This allows the interrogation of the biochemical processes occurring in individual plaques in humans, and opens the possibility of personalized medicine.
- Proteolytic and oxidant-mediated modification of the extracellular matrix of the artery wall, and its role in atherosclerosis.
Recent data indicate that oxidants can activate (switch-on) the activity of powerful proteolytic enzymes that damage the structure of the artery wall. This project is examining how this occurs and which enzymes are the key players in extracellular matrix damage.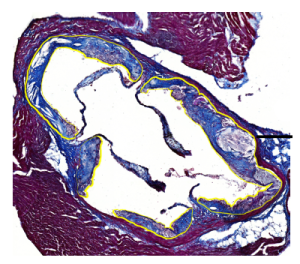
- Myeloperoxidase, inflammation and cardiovascular disease.
Activated leukocytes which accumulate at sites of inflammation, including within the artery wall, release the heme enzyme myeloperoxidase. Elevated levels of this enzyme and the species that it generates are strongly associated with tissue damage. We are examining how this enzyme damages the cells and extracellular matrix of arteries.
- Protection against myeloperoxidase-mediated damage by small anions and enzyme inhibitors
Inhibition of myeloperoxidase, or provision of alternative substrates, may modulate the damage induced by this enzyme within the inflamed artery wall. We have shown that a number of small anions can alter the extent of damage, at concentrations that can be readily achieved in humans.
- Kinetics and mechanisms of oxidative damage to proteins induced by hypochlorous acid, peroxynitrous acid, singlet oxygen, electrophiles, peroxides, sugars and metal ions.
Understanding how oxidants react and target proteins, and individual sites within these, is critical to understanding biological damage and the development of protective strategies.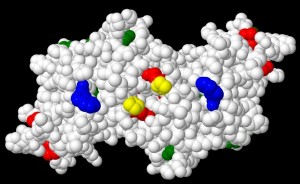
- Novel selenium- and sulfur-based antioxidants
A number of enzymes have selenium and sulfur atoms in their active sites which make these highly efficient protective agents against damage. This project aims to develop low molecular mass analogues to protect against, or modulate disease.
- Role of molecular crowding in modulating protein structure, function and oxidation
Tissues and cells are highly crowded environments, but many model systems use simple dilute systems to model these complex systems. Our data indicate that the high concentrations of (macro)molecules in cells and biological fluids have profound effects on protein structure and function, cellular signaling, oxidation reactions and aggregation. Understanding these effects is critical to understanding cellular biochemistry.
- Detection, characterization and quantification of protein cross-links
Many human diseases including cardiovascular and neurodegenerative conditions (e.g. Alzheimer’s and Parkinson’s diseases) are characterized by the accumulation of large protein aggregates. The nature of these species and the cross-links between proteins that drive aggregate formation are poorly understood. We are developing new approaches to detect and identify these species, and are examining aggregate formation in cells, tissues, food samples and medicines.
Please contact Michael Davies, davies@sund.ku.dk, if you are interested in any of these projects, or other opportunities.
We collaborate with a large number of international and national researchers including the following:
- Prof. Clare Hawkins (BMI, Copenhagen, Denmark)
- Prof. Ernst Malle, Dr. Astrid Hammer (University of Graz, Austria)
- Prof. Carl Schiesser (Seleno Therapeutics, Melbourne, Australia)
- Prof. Henning Bundgaard (Rigshospitalet)
- Prof. Kasper Iversen (Herlev and Gentofte Hospitals)
- Prof. Peter Ogilby (Aarhus, Denmark)
- Prof Camilo Lopez Alarcon (Pontificia Universidad Católica de Chile, Santiago, Chile)
- Prof. Jonas Eiberg (Rigshospitalet)
- Prof. Tim Resch (Rigshospitalet)
- Dr. Marta Ignasiak (Adam Mickiewicz University, Poland)
- Prof. Daniel Otzen (Aarhus University, Denmark)
- Prof. Rafael Radi (Universidad de la República, Uruguay)
- Prof. Trond Ulven (University of Copenhagen)
- Prof. Christian Olsen (University of Copenhagen)
- Assoc. Prof. Thomas Jørgensen (University of Southern Denmark)
- Assoc. Prof. Adelina Rogowska-Wrzesinska (University of Southern Denmark)
- Assoc. Prof. Thomas Jepps (University of Copenhagen)
- Prof. Alicia Lundby (University of Copenhagen)
- Assoc. Prof. Rebecca Miller (University of Copenhagen)
- Assoc. Prof. Christina Christoffersen (University of Copenhagen)
- Assoc. Prof. Pontus Gourdan (University of Copenhagen)
The Protein Oxidation Group has received, and continues to be funded by, grants from a number of private and public foundations including: Novo Nordisk Fonden (Laureate Grants x 2 and Project grants), Lundbeck Fonden, Danish Council for Independent Research, La Caixa Foundation, Augustinus Foundation, AP Møller Foundation, Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis' Legat.
Prof. Davies has a number of senior roles in scientific societies and organizations including:
- 2022-present: Joint Editor in Chief, Redox Biochemistry and Medicine
- 2022-present: Scientific Integrity and Ethics Editor for Redox Biology and Advances in Redox Research
- 2019-2020: President of the Society for Free Radical Research Europe
- 2020: Chair: Society for Redox Biology and Medicine – SFRR-Europe Publications Outreach committee
- 2017-2018: President-Elect of the Society for Free Radical Research (Europe)
- 2016-2019: Senior Scientific Advisor, EU H2020 ITN-ETN MASSTRPLAN program
- 2013-2014: President of the Society for Free Radical Research International
- 2012-2013: President-Elect, Society for Free Radical Research International
- 2009-2018: Editor-in-Chief, Free Radical Research
- 2009-present: Associate Editor, Photochemistry and Photobiology
- 2008-2011: Vice-President, International EPR Society
- 2007-2010: Secretary-General, Society for Free Radical Research International
- 2005-2008: Council member, American Society for Photobiology
- 2001-2003: President, Society for Free Radical Research (Australasia)
- Director, Seleno Therapeutics Pty. Ltd
Group leader
Michael Davies
Professor
Phone +45 2364 9445
davies@sund.ku.dk
ORCID: 0000-0002-5196-6919
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Christine Chuang | Assistant Professor | +4535337852 | |
| Karen Chuxian Yang-Jensen | PhD Fellow | ||
| Kathrine Væver Jokumsen | PhD Fellow | ||
| Lasse Gøbel Lorentzen | Guest Researcher | +4535327449 | |
| Patricia Capillas Herrero | PhD Fellow | ||
| Per Mårten Hägglund | Special Consultant | +4535332764 | |
| Yibo Gao | PhD Fellow | +4535328953 |

