Ober Group: Liver Development and Regeneration
"Our group aims to understand how a functional organ is built from a population of self-organising progenitor cells. In particular, which morphodynamic processes control initial progenitor migration and subsequently their differentiation into epithelial unites organized into a functional architecture. We apply high-resolution imaging together with genetic and cell biological techniques to monitor progenitor cell dynamics in liver development and regeneration.
The ability of the liver to carry out the many diverse metabolic functions essential for body homeostasis depends on its specialized cell types and their organization within the tissue. In the embryo, progenitor cells differentiate into mature organs. This includes the transition from migratory progenitor cells into functional epithelial units consisting of polarized hepatocytes and biliary ducts. This is accompanied by dramatic changes in tissue morphology, which are driven by dynamic cell rearrangements and cell- and tissue interactions coordinated with the growth of the whole organ and embryo. How individual progenitor cells assemble a functional organ in the developing embryo is a fundamental question and remains poorly understood. Whether similar morphogenetic cell behaviours are crucial for tissue regeneration after injury is another key problem.
We combine a wide range of molecular, genetic and modern imaging techniques to identify the mechanisms driving progenitor cell positioning and differentiation in liver development and regeneration with the ultimate goal to uncover the basis of human developmental disorders and directing tissue-engineering approaches for therapeutic purposes.
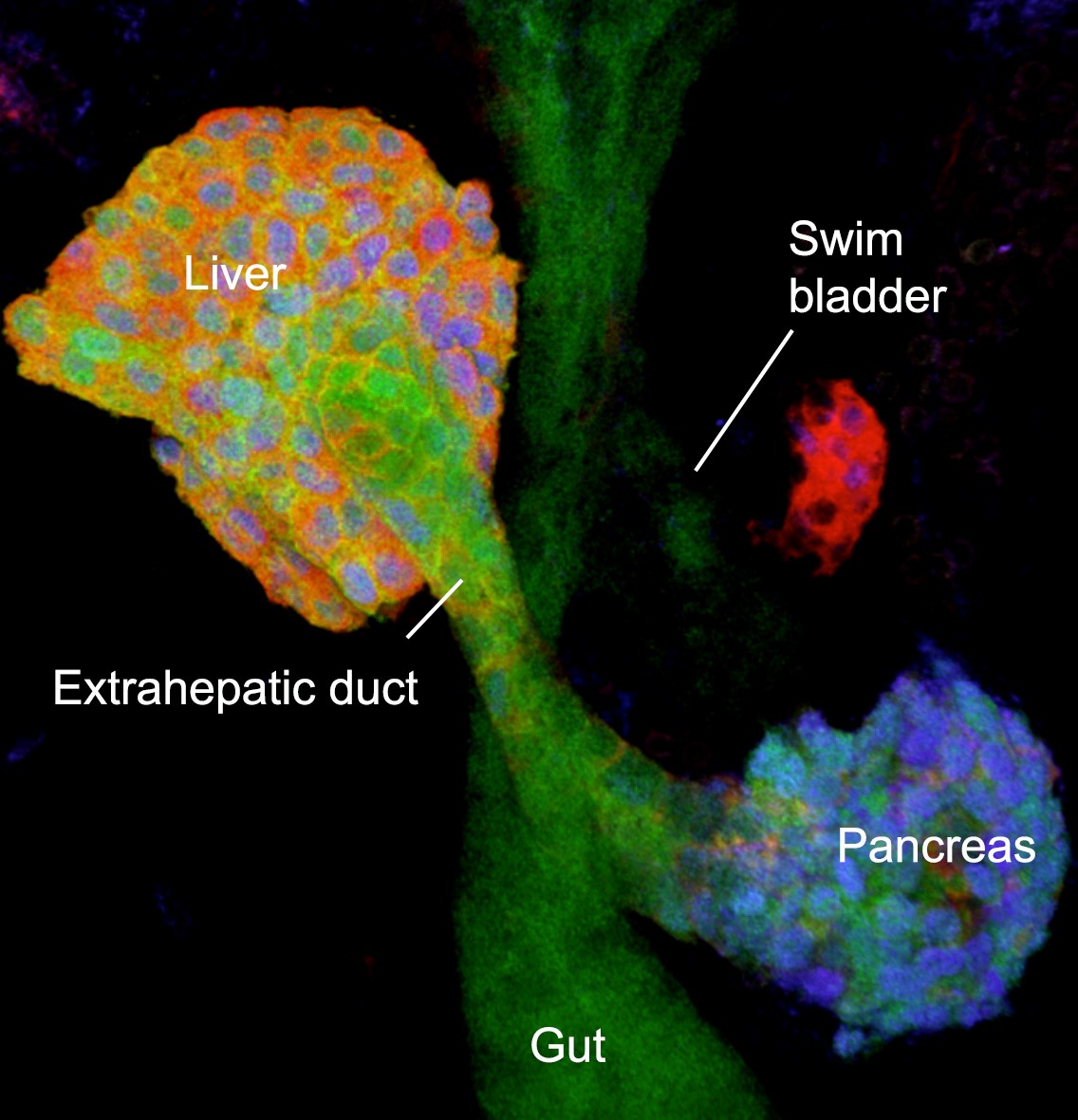 Foregut organs: overview of the digestive organs of a 2 day old zebrafish embryo (confocal projection)..
Foregut organs: overview of the digestive organs of a 2 day old zebrafish embryo (confocal projection)..
We use zebrafish as a model, which is ideal for studying tissue morphogenesis, because embryos and larvae are transparent and cell behaviours can be visualized at single cell resolution in the living animal. For this, we have generated new transgenic tools that enable the monitoring and tracking of individual cells in 4D and by combining them with different genetic, cell biological, quantitative imaging and biophysics approaches allow to analyse liver morphogenesis.
 The liver of a 5 day old zebrafish is marked green by transgenic lfabp:GFP-expression.
The liver of a 5 day old zebrafish is marked green by transgenic lfabp:GFP-expression.
Ongoing projects:
- Liver bud formation: cell biology and biomechanics of progenitor migration
- Molecular and cellular mechanisms of extrahepatic duct formation
- Morphodynamic cell behaviours generating a functional liver architecture
- Morphogenetic processes underlying liver regeneration
Selected publications:
Caviglia S*, Unterweger I.A.*, Gasiunaite A,. Vanoosthuyse, A.E., Cutrale, F., Trinh, L.A., Fraser S.E.,Neuhauss S.C.F., and Ober E.A.§. (2022) FRaeppli, a multispectral imaging toolbox for lineage tracing and dense tissue analysis. Development 149(16):dev199615. doi: 10.1242/dev.199615
Dzementsei, A., Barooji, Y., Ober, E. A.& and Oddershede, L.B.& (2022) Foregut organ progenitors and their niche display distinct viscoelastic properties in vivo during early morphogenesis stages. Communications Biology 5(1):402. doi: 10.1038/s42003-022-03349-1.
Ellman, D. G., Mathiesen, S. B., Slaiman, I. M., Andersen, K. S., Hofmeister, W., Ober, E. A. and Andersen, D. C. (2021) Apex resection in zebrafish (Danio rerio) as a model of heart regeneration: Video-assisted guide. Int. J. Mol. Sci 22(11):5865. doi: 10.3390/ijms22115865
Thestrup, M. I., Caviglia,, S., Cayuso, J., Heyne, R. S. L., Satriano, L., Ahmad, R., Hofmeister, W., Andersen, J. B., Wilkinson, D. G. and Ober, E. A. (2019) A morphogenetic EphB/EphrinB code controls hepatopancreatic duct formation. Nature Communications 10(1):5220; doi: 10.1038/s41467-019-13149-7
Lonowski, L.A., Narimatsu, Y., Riaz, A., Delay, C.E., Yang, Z., Niola, F., Duda, K., Ober, E. A., Clausen, H., Wandall, H.H., Hansen, S.H., Bennett, E.P. and Froedin, M. (2016) Genome editing using FACS enrichment of nuclease expressing cells and Indel Detection by Amplicon Analysis (IDAA). Nat. Protoc. 12(3):581-603.
Cayuso, J., Dzementsei, A., Fischer, J. C., Karemore, G., Caviglia, S., Bartholdson, J., Wright, G. J. & Ober E. A. EphrinB1/EphB3b coordinate bidirectional epithelial-mesenchymal interactions controlling liver morphogenesis and laterality. Dev Cell. 39, 316-328 (2016).
Koltowska K, Lagendijk AK, Pichol-Thievend C, Fischer JC, Francois M, Ober EA, Yap AS, Hogan BM. (2015) Vegfc Regulates Bipotential Precursor Division and Prox1 Expression to Promote Lymphatic Identity in Zebrafish. Cell Reports 13(9):1828-41.
Lonowski, L.A., Narimatsu, Y., Riaz, A., Delay, C.E., Yang, Z., Niola, F., Duda, K., Ober, E.A., Clausen, H., Wandall, H., Hansen, S.H., Bennett, E.P., and Frödin, M. (2017). Genome editing using FACS enrichment of nuclease-expressing cells and indel detection by amplicon analysis. Nature Protocols 12, 581-603, doi:10.1038/nprot.2016.165.
Wang, S., Miller, S.R., Ober, E.A. and Sadler, K.C. (2017). Making it new again: Insight into liver development, regeneration, and disease from zebrafish research. Current Topics in Developmental Biology 124, 161-195, doi:10.1016/bs.ctdb.2016.11.012.
Cayuso, J., Dzementsei, A., Fischer, J.C., Karemore, G., Caviglia, S., Bartholdson, J., Wright, G.J. and Ober E.A. (2016). EphrinB1/EphB3b Coordinate Bidirectional Epithelial-Mesenchymal Interactions Controlling Liver Morphogenesis and Laterality. Developmental Cell 39, 316-328, doi:10.1016/j.devcel.2016.10.009. Recommended by the faculty of 1000
Ober, E.A. and Grapin-Botton, A. (2015). At new heights - endodermal lineages in development and disease. Development 142, 1912-1917, doi: 10.1242/dev.121095.
Koltowska, K., Apitz, H., Stamataki, D., Hirst, E.M.A., Verkade, H., Salecker, I., and Ober, E.A. (2013). Ssrp1a controls organogenesis by promoting cell cycle progression and RNA synthesis. Development 140, 1912-1918, doi:10.1242/dev.093583.
Poulain, M. and Ober, E.A. (2011). Interplay between Wnt2 and Wnt2bb controls multiple steps of early foregut-derived organ development. Development 138, 3557-3568, doi:10.1242/dev.055921.
Liver Morphology