Link to crowdfunding
Sylow Group - Molecular Metabolism in Cancer and Ageing

By employing cellular, murine, and human models and samples, our research aims to uncover novel concepts and pharmacological targets to ameliorate insulin resistance and cachexia in various age-related afflictions such as cancer, sarcopenia, and diabetes.
With muscle wasting and insulin resistance being central features of cancer cachexia, ageing, and diabetes, we are uncovering the pathology of these conditions at the molecular level.
One area of particular interest is investigating the potential for exercise training to ameliorate or even reverse muscle wasting and metabolic aberrations.
Specifically, we focus upon the intricate molecular pathways that govern the maintenance of cellular homeostasis, muscle mass and metabolism, mitochondrial biology, mitochondrial RNA stability, GLUT4 translocation, glucose uptake, insulin sensitivity, Rho GTPases, and adaptations arising from exercise training.

We investigate the regulatory mechanisms governing muscle mass and insulin signaling in various health conditions, including ageing, cancer, and Type 2 diabetes.
To achieve this objective, we employ several models and methods, such as in vitro muscle and fat cell systems, cloning, preclinical mouse models of obesity and cancer, recombinant adeno-associated virus skeletal muscle transfections, inducible conditional knockout mice, and human subjects and tissue materials.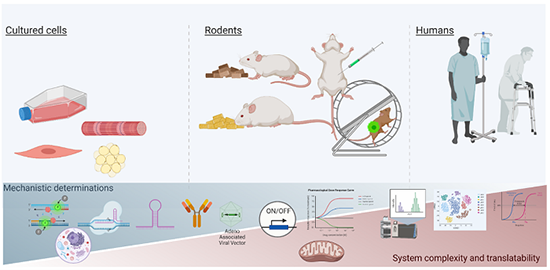
Laboratory LEAF certified standard for sustainable laboratory operations:
Laboratory-based research is highly resource-intensive. Labs contribute to 2% of global plastic waste and consume 3-10 times more energy per meter squared than an average office.
By joining the LEAF program, we try to reduce our carbon emissions and create an environment that supports research quality.
Our laboratories are awarded the Gold level

- Independent Research Fund - Danmarks Frie Forskningsfond
- Danish Cancer Society –Kræftens Bekæmpelse
- Helsefonden
- Aase og Ejnar Danielsens Fond
- Lundbeck Foundation
- Danish Diabetes and Endocrine Academy
- Novo Nordisk Foundation
- Kong Christian den Tiendes Fond
- Innovation Fund Denmark
- Carlsberg Foundation
- European Foundation for the Study of Diabetes
Research theme 1:
Healthy after cancer
This research theme aims to identify the molecular causes of metabolic dysfunctions in cancer with a focus on skeletal muscle and adipose tissue metabolism. Cancer causes skeletal muscle insulin resistance and elevates type 2 diabetes incidence, which in turn increased cancer mortality. Moreover, cancer patients are at high risk of developing muscle loss (cachexia). Insulin resistance and cachexia increase treatment toxicity and reduce patient survival but there is currently no treatment. Elucidation of new pathways that coordinate muscle insulin resistance and cachexia is a major challenge and an untapped opportunity for therapeutics in cancer.
It is well-known that metabolic perturbations, such as obesity and type 2 diabetes increase the risk of developing cancer. Additionally, cancer patients with diabetes or obesity have a much higher risk of death and increased likelihood of cancer recurrence than lean otherwise healthy cancer patients.

In particular, breast and lung cancer are associated with impaired metabolic regulation and insulin resistance. Our current research examines the presence and molecular causes of cancer-induced metabolic dysregulation in cell models, mouse models, as well as people with cancer. We investigate the role of physical activity, inflammation, altered fat metabolism, and intracellular molecular signaling in cancer-associated insulin resistance in muscle and fat.
Research theme 2:
Identification of muscle molecules that orchestrate the beneficial effects of exercise
Exercise is the most effective means to prevent non-communicable diseases and promote health. This project identifies novel muscle molecules that orchestrate the beneficial effects of exercise.
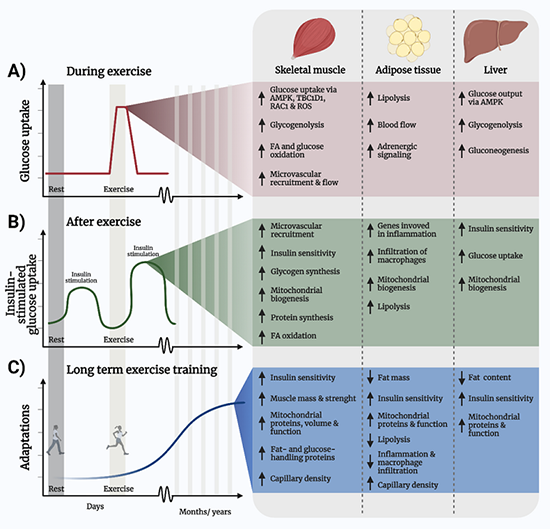
Exercise acutely promotes health benefits, such as promoting skeletal muscle glucose uptake, increasing energy utilization, as well as increasing insulin sensitivity in the hours after the exercise bout. In addition to the acute effects of one exercise bout, regular exercise training elicits longer-term benefits, such as increased mitochondria mass and function, amplified capillarization, upregulation of metabolically important proteins, optimization of substrate utilization, and muscle hypertrophy.
All these adaptations benefit our health. This project investigates the intracellular molecular mechanisms regulated by exercise that mediate these effects with a focus on mitochondrial and muscle mass regulation.
Research theme 3:
Metabolism and muscle wasting in ageing and disease
Ageing and certain diseases, such as cancer, often result in loss of muscle mass. Muscle wasting can cause dysregulated metabolism due to reduced muscle mass. Additionally, the remaining muscle often does not function optimally metabolically. Thus ageing- (sarcopenia) and cancer- (cachexia) related muscle wasting is often associated with poor glycemic regulation and skeletal muscle insulin resistance.
Our research is exploring how cancer and old age affect muscle mass and metabolic processes in muscle and other metabolically active tissues.
In that context, we are investigating the molecular mechanisms by which exercise fosters enhanced muscle function and improves health. Our hope is that identifying the molecular causes of age-, cancer-, and diabetes related muscle decline and the mechanisms by which exercise benefit those conditions, we can develop new and better strategies for treating such conditions.
Research theme 4:
Rho GTPases as novel players in metabolism and muscle mass regulation
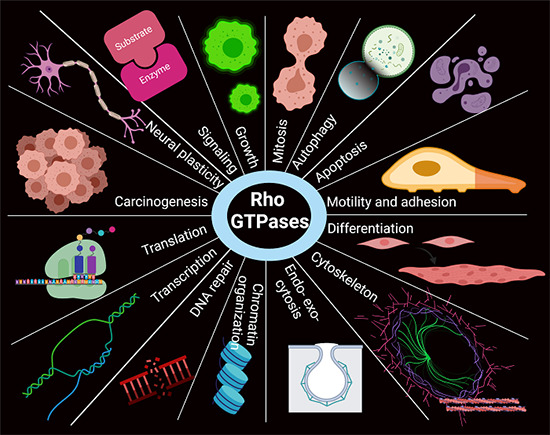
Rho GTPases are small molecules that regulate many different processes in the cells, such as cell division and vesicle transport. We are investigating how these molecules are regulated in skeletal muscle and what role they play in muscle metabolism.
We are studying the Rho GTPases expressed in skeletal muscle and have identified Rac1 as a critical regulator of glucose uptake in skeletal muscle, which is a novel player in skeletal muscle insulin resistance in conditions of obesity and type 2 diabetes. This project is further elucidating novel regulators of the Rho GTPase pathways, including the chaperone, GDIa, which is an inhibitor Rho GTPases.
This research theme explores the role for Rho GTPases in skeletal muscle mass and insulin sensitivity regulation and identify their up- and down-stream regulators.
We strive for excellence, equity, diversity, and inclusion. We work with the common goal to identify mechanisms that, at the molecular level, regulate muscle mass, function, and metabolism in health and disease.
We welcome any background, race, ethnicity, gender, age, religion, and identity. We celebrate multiple approaches and points of view. Inclusion is how we unleash the power of diversity. Diversity is the prerequisite for innovation, creativity, and excellence.
We follow the University’s policy and do not tolerate any forms of bullying, sexual harassment or other kinds of offensive behavior towards anyone. We are committed to fostering belonging and empowerment at work for all.
Podcast
Kan vi løbe fra kræften? (2025)
Obesity, mobility, exercise and cancer are related (2025)
Lykke gransker mekanismerne bag musklernes stofskifte og fysiologi (2025)
Inside Exercise: Exercise preserves muscle and metabolism during cancer with Lykke Sylow (2024)
Articles
Lykke Sylow er Månedens Forsker (2025)
Hvordan træning beskytter musklens energifabrikker (2025)
Forbes: Is There A Drug To Help You Lose Fat And Gain Muscle Without Exercise? (2024)
Science News: Mangel på gen overrasker: Gør det umuligt at tage på (2023)
Science News: Insulinresistens spiller vigtig rolle ved kræft (2023)
Dagens Medicin: Dansk forskning: Metabolistisk kontrol bør være et mål ved behandlingen af kræft (2023)
Science News: Kræftpatienter har forhøjet risiko for at udvikle type 2-diabetes (2022)
Science News: Et uudforsket molekyle kan måske tøjle diabetes (2020)
Group members
| Name | Title | Phone | |
|---|---|---|---|
| Andrea Irazoki | Postdoc Marie Curie | +4535324937 | |
| Anna Martha Hammershøi Madsen | PhD Fellow | +4535325897 | |
| Camilla Lund | Guest Researcher | +4550928304 | |
| Caroline Manon Markmann Bentsen | Master Student | ||
| Christian Strini Carl | Postdoc | ||
| Edmund Hugh Battey | Postdoc | ||
| Emma Helene Frank Larsen | Research Assistant | ||
| Erik A. Richter | Guest Researcher | +4535321626 | |
| Karina Rasmussen | Laboratory Technician | +4535327485 | |
| Lisbeth Liliendal Valbjørn Møller | Postdoc | +4535331114 | |
| Lykke Sylow | Associate Professor - Promotion Programme | +4535321767 | |
| Michala Carlsson | PhD Fellow | +4535326514 | |
| Nicoline Resen Andersen | Academic Staff | +4535321765 | |
| Steffen Henning Raun | Postdoc | +4535334265 | |
| Tang Cam Phung Pham | Postdoc | +4535330487 | |
| Zakarias Kefil Jensen Ogueboule | PhD Fellow | +4535333737 |

Group Leader
Lykke Sylow
Associate Professor
Department of Biomedical Sciences
Endocrinology and Metabolism
Maersk Tower - 7.2
Phone +45 2095 5250
lykkesylow@sund.ku.dk
ORCID: 0000-0003-0905-5932
 |
 |